张明华1,朱曼1,陈超1,裴斐1,石莉1,孙艳2,*,郭代红2,*
1中国人民解放军总医院,药品保障中心,临床药学室,100853
2中国人民解放军总医院,药品保障中心,100853
*通讯作者: guodh301@163.com 或者 301sunyan@sina.cn
摘要
MLN4924是一种选择性的NAE抑制剂,希望成为肿瘤治疗药物。MLN4924能够抑制Cullin-RING泛素连接酶活性,上调其底物,干扰肿瘤细胞的S期DNA合成,最终导致肿瘤细胞的凋亡。Cullin-RING的泛素连接酶活性需要Neddylation体系的修饰。HECT类泛素连接酶ITCH在肿瘤的发展过程中具有重要的作用,可能与Neddylation也有密切的关系。探索ITCH的泛素连接酶作用机制就可能有助于更全面地理解MLN4924的治疗机制。我们发现,Nedd8的修饰可激活ITCH的泛素连接酶活性,促进对p73等底物的降解,其活性可被MLN4924抑制。ITCH在结直肠肿瘤中显著高表达。敲低ITCH后结直肠癌细胞系的增殖和迁移能力受到显著的抑制,且与MLN4924存在协同作用。ITCH的激活机制及其在肿瘤中的调控作用使其有可能成为新的肿瘤药物作用靶点。
关键词:蛋白质降解,泛素连接酶, Neddylation,ITCH,结直肠癌
Targeting HECT-type E3 ligase ITCH for Inactivation by MLN4924 in Colorectal Cancer Cell Line
Minghua Zhang1, Man Zhu1, Chao Chen1, Fei Pei1, Li Shi1, Yan Sun2,*, Daihong Guo2,*
1Department of Clinical Pharmacy, Center of Pharmacy, Chinese PLA General Hospital, Beijing 100853, China
2 Center of Pharmacy, Chinese PLA General Hospital, Beijing 100853, China
*Correspondence: guodh301@163.com or 301sunyan@sina.cn
Abstract
MLN4924 is a potent and selective inhibitor of NAE, holding promise for the treatment of cancer. The mechanism of MLN4924 was thought to be disrupting cullin-RING ligase-mediated protein turnover leading to the deregulation of S-phase DNA synthesis and apoptotic death in human tumour. Cullin-RING E3s are activated via modification by the ubiquitin-like protein Nedd8 in a process called Neddylation. Neddylation maybe also involved in the activation of HECT-type E3 ligase ITCH, wich plays an important role in tumorigenicity. So, deciphering the activation mechanisms of ITCH is critical for understanding the mechanism of MLN4924 in cancer treatment. Here, we show that ITCH is activated by Neddylation, promoting its ubiquitination ligase activity and the degredation of p73, wich can be inhibited byMLN4924. ITCH expression is elevated in colorectal cancer tissues compared with adjacent tissues. Further experiments indicated that knockdown of ITCH in HCT116 cells resulted in impaired cell growth and migration. And there is a synergy between shRNA-ITCH and MLN4924. These findings shed new light on the activation of ITCH and its potential role to be an anti-tumour drug target.
Keywords: protein degradation; ubiquitin ligase; Neddylation; ITCH; colorectal cancer
以色列人阿龙·切哈诺沃(AaronCiechanover)发现了泛素蛋白酶体系统,并因此在2004年与阿夫拉姆·赫什科(Avram Hershko)、欧文·罗斯(Irwin Rose)一道获得诺贝尔化学奖[1]。泛素蛋白酶体系统的降解过程需要三种酶参与。首先,泛素活化酶(E1)消耗ATP,并腺苷酸化一个泛素分子。接着,泛素转运到E1的活性中心,与此同时,E1腺苷酸化第二个泛素[2]。随后泛素分子被转移到泛素结合酶(E2),与E2的半胱氨酸残基结合。最后,泛素连接酶(E3)将泛素分子从E2转移到高特异性的蛋白底物上,只有四个泛素分子组成的泛素连结合到底物分子上后,这个底物蛋白才会被蛋白酶体识别和降解[3]。所以,底物的特异性由泛素连接酶决定[4]。
泛素蛋白酶体系统负责调控多种信号通路中关键蛋白因子的降解[5]。而且也有越来越多的证据表明泛素蛋白酶体系统参与了肿瘤的发展。肿瘤中泛素连接酶的表达扩增或缺失已经多有报道[6, 7]。很多泛素连接酶也已经被鉴定为癌基因(比如BRCA1和 Fbw7)或抑癌基因(比如 Mdm2 和 Skp2)。在多数结直肠癌中,由于APC失活,导致泛素蛋白酶体降解β-连环蛋白能力下降,从而导致肿瘤细胞增殖失控。Nedd4家族泛素连接酶ITCH与肿瘤的关系也非常密切[8-11]。一些调控肿瘤的重要分子,比如p53,Smad4和k-ras家族的某些成员等,也受到了泛素连接酶的调控。在泛素化反应的体系中,泛素连接酶具有底物识别的特异性,从而有可能成为肿瘤靶向性治疗的更好的靶标。
有些分子在结构上和泛素分子比较相似,也能够象泛素一样被修饰到底物上,这些分子统称为类泛素分子。目前已经发现的类泛素分子至少有17种[12]。其中Nedd8 (neural-precursor-cell-expressed developmentally down-regulated 8)是与泛素关系最为密切的一个。它与泛素在一级序列上有80%的相似度,在高级结构上也非常相似[13]。催化Nedd8结合到底物上的一系列酶也和泛素化系统非常类似[14]。能够被Nedd8修饰的底物分子比泛素要少,研究较多的底物是Cullin类分子。Cullin类分子是CRLs (cullin-RING ubiquitin ligases)类泛素连接酶的支架分子。Nedd8修饰到Cullin类分子上,使其空间构象发生改变,同时阻止Cand1对Cullin的抑制作用[15, 16]。
由千禧年药业有限公司(Millennium Pharmaceuticals, Inc.)和强生公司联合开发的多发性骨髓瘤治疗药物Velcade,就是一种蛋白酶体抑制剂,该药物已经成功用于临床治疗恶性骨髓瘤,而且对慢性淋巴细胞白血病的治疗也已经处于临床实验阶段[17, 18]。Velcade的成功应用提示泛素蛋白酶体系统中的关键酶可以作为肿瘤等疾病的靶点,用于开发新的肿瘤靶向性药物[19, 20]。但是Velcade对泛素蛋白酶体系统的抑制是广谱的,而不是特异的靶向肿瘤细胞,具有相对比较广泛的不良反应。千禧年药业新一代泛素化抑制剂MLN4924进一步明确了作用靶点,缩小了抑制范围,对结直肠癌获得了显著的疗效,与Velcade相比,明显地减少了不良反应[21]。目前认为,MLN4924可能就是通过高效地具有选择性地抑制Nedd8活化酶(NAE),干扰cullin-RING泛素连接酶介导的蛋白质的转归(protein turnover),从而导致肿瘤细胞周期S期DNA合成失调,最终导致肿瘤细胞凋亡。
然而,最近几年发现,Neddylation和泛素化两条通路存在更多的交叉[22-24]。并且这种交叉在细胞内比较普遍,比如在各种应激、自由的泛素分子减少时、Nedd8分子增加时(比如Nedd8过表达时)、蛋白酶体受到抑制等条件下,都会发生。在细胞内过表达Nedd8时,甚至会发现,本应完全由泛素组成的泛素链,中间的某些泛素分子可能会被Nedd8所取代,成为由泛素和Nedd8共同组成的一条“类泛素蛋白链”[22]。还有一些连接酶既是Neddylation系统的E3,又是泛素化系统的E3。所以,Nedd8修饰某些底物之后,作用就很难一概而论,需要在具体通路、具体分子和具体条件下进行分析;MLN4924对肿瘤的作用机制,也就增加了很多可能性。在前期的研究中我们发现,HECT类泛素连接酶ITCH可能就处于两个通路的交叉点上,其活性也受到MLN4924的调控。
1. 材料与方法
1.1 材料与试剂
Nedd8、ITCH的表达序列通过PCR构建到相应表达载体上。pGEX-4T-2载体购自Amersham公司。pCMV-Myc真核表达载体购自Clontech公司。MLN4924购自Active BioChem公司。Pyrobest DNA Polymerase、DL-2000 DNA marker、dNTP、DL-5000 DNA marker、T4 DNA连接酶和限制性内切酶均购买于TaKaRa公司;逆转录试剂盒购买于Toyobo公司;质粒提取试剂盒、凝胶回收试剂盒均购买于Promega公司;RPMI 1640、DMEM、DMEM/F12、胎牛血清及双抗及胰酶购买于Hyclone公司;感受态细胞BL21、DH5α、GSTK抗体购自TIANGEN;TRIZOL、Lipofectamine2000、FITC标记的羊抗鼠抗体购买于Invitrogen公司;NC膜、尼龙膜购买于Amersham公司;Western blot实验显色试剂盒购买于Pierce公司。
1.2 免疫印迹
除去培养液,1ml的PBS洗涤2次。滴入裂解液以及等量的2×loading并混匀。.沸水煮0-15分钟并1000g离心4分钟。进行SDS-PAGE,并转膜。含有5%脱脂牛奶的TBST慢摇1h。封口膜包裹,并于4℃过夜。使用TBST洗三次,每次10min左右。在室温中孵育二抗45分钟,并TBST洗涤三次,每次10分钟左右,加发光底物并进行曝光显影。
1.3 免疫组化
将白片进行脱蜡:2×浓度的二甲苯15min。 水化的顺序如下:100%乙醇 3min,95%乙醇 3min,90%乙醇 3min,80%乙醇 3min;重复步骤b,用蒸馏水冲干净切片。除去内源过氧化物酶:使用3% H2O2 室温孵育10min(PBS配置,注意现用现配,隔绝氧),PBS洗2min X 3。 抗原修复:向抗原修复盒中灌满PBS溶液,在微波炉中中火加热5min,在PBS将沸未沸时(95度),置入切片,换成小火5min加热,后取出修复盒,冷却至室温后,约需2小时,取出切片。Trition-100破膜:将切片平铺于冰上,滴上PBS配置的Triton X-100 (0.5%-1%),20min。完毕后PBS清洗洗3遍,血清封闭37℃1小时。一抗孵育:一抗用3% bsa-pbs配置,湿盒中37℃孵育2小时或4度过夜。完毕后PBS洗3遍。使用中山金桥二步法试剂盒。Dab显色。
1.4 细胞生存和迁移实验
利用假病毒颗粒感染细胞,获得稳定株,传代铺入24孔板。每隔24h,胰酶消化吹散细胞,取10ul混悬液加入一次性计数板,电子计数。
于Transwell孔中加入无血清培养基,下层培养皿中加入含5%血清的培养基,将稳定株细胞铺入transwell孔中培养,24小时后取出transwell孔,清洗干净后甲醇固定30分钟,结晶紫染色20分钟,清洗晾干后扫描。
2. 结果
2.1 Nedd8与ITCH存在相互作用,且Nedd8能够共价修饰ITCH,这种修饰依赖于Neddylation体系。
在结肠癌细胞系HCT116中,我们利用内源的ITCH抗体检测Nedd8抗体的免疫沉淀样品,发现Nedd8与ITCH存在相互作用(Fig. 1A)。纯外源pull down实验结果显示ITCH和Nedd8存在直接的相互作用(Fig. 1B)。在HCT116细胞系中免疫共沉淀后,利用Nedd8抗体检测共纯化的ITCH蛋白,我们发现Nedd8对ITCH的修饰结果为连续的条带状,且这种修饰受到MLN4924的抑制(Fig. 1C)。此外,利用siRNA干扰Neddylation E1的亚基Uba3后,Nedd8对ITCH的修饰同样显著降低(Fig. 1D)。
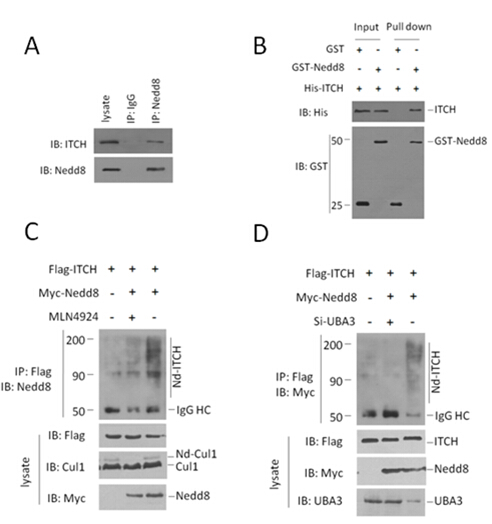
Fig.1 (A) Endogenous interaction of ITCH and Nedd8 in HCT116 cells. Both the cell lysates and immunoprecipitates were analysed by western blotting with the indicated antibodies. (B) GST pull-down assay was performed to detect the direct interaction between ITCH and Nedd8. Both input and pull-down samples were subjected to immunoblotting (IB) with anti-His and anti-GST antibodies. Input represents 10% of that used for pull-down. (C,D) ITCH was neddylated in HCT116 cells. This neddylation was significantly attenuated by NAE inhibitor MLN4924, or depletion of Uba3 (one subunit of NAE). The indicated plasmids or siRNAs were transfected into HCT116 cells. Flag antibody-immunoprecipitated ITCH proteins were analyzed by immunoblotting with anti-Myc antibody to detect conjugated Nedd8.
2.2 Nedd8的修饰促进ITCH对底物的泛素化活性。
ITCH的泛素化底物包含P73、P63、LAPTM5和RASSF5/NORE1等重要的分子,并可能通过这些底物参与肿瘤发展的调控[10, 11, 25]。在HCT116细胞系中过表达外源Nedd8、ITCH、P73和泛素,利用外源P73抗体免疫沉淀后,检测外源Ub,我们发现,Nedd8可促进ITCH对P73的泛素化水平(Fig . 2A)。同时,Nedd8能够促进ITCH对P73的降解,而且我们发现与其他HECT类泛素化连接酶类似,ITCH在降解底物时自身降解也在加速(Fig . 2B)。
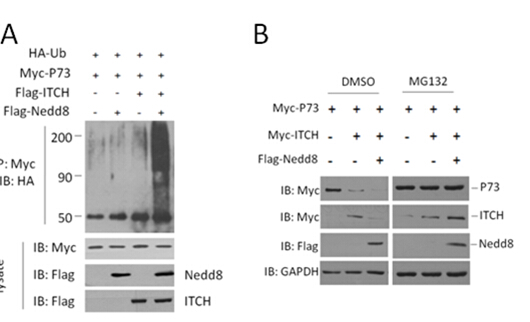
Fig.2 (A) Nedd8 promotes the ubiquitination activity of ITCH in HCT116 cells. The indicated plasmids were transfected into HCT116 cells. Myc antibody immunoprecipitated Ub proteins were analyzed by immunoblotting with anti-HA antibody to detect conjugated Ub. (B) P73, ITCH and Nedd8 were co-transfected into HCT116 cells. Cell lysates were immunoblotted to detect p73 levels.
2.3 ITCH在结直肠肿瘤中显著高表达,在肿瘤细胞分裂期与中心体和微管存在共定位。
为探索ITCH在结直肠肿瘤中的表达水平,利用免疫组化技术在80例结直肠肿瘤样本的蜡块切片中检测ITCH的表达水平。我们发现,肿瘤组织中ITCH的蛋白水平显著高于同一患者的癌旁组织(Fig. 3A)。ITCH主要定位于细胞质,且分布不均匀,分裂间期细胞中,ITCH的染色往往聚集成团块状,靠近细胞核(Fig. 3B)。为了进一步检测ITCH的亚细胞定位,利用内源ITCH抗体、α-tublin抗体和DAPI,在Hela细胞中进行激光共聚焦,我们发现,在细胞的分裂期,ITCH主要定位于中心体(Fig. 3C)。


Fig.3 ITCH overexpressed in colorectal cancer and locallized in centrosomes in mitosis. (A) ITCH expression scores are shown as box plots, with the horizontal lines representing the median; the bottom and top of the boxes representing the 25th and 75th percentiles, respectively; and the vertical bars representing the range of data. Colorectal cancer tissues were compared with matched adjacent normal tissues using the Wilcoxon test. n = 80. Representative images from immunohistochemical staining of ITCH expression of the same tumor from three cases. Scale bars, 100um.(B). Colorectal cancer tissue stained with antibodies to the ITCH protein. Bars, (left) 40 um, (right) 20um.(C) Representative images of ITCH protein localization in metaphase. The cells were stained with 4,6-diamidino-2-phenylindole(DAPI; for DNA; blue), anti-tubulin (microtubules; red) and ITCH antibody (kinetochores; green). Scale bar, 10 um.
2.4 ITCH在结直肠肿瘤中显著高表达,在肿瘤细胞分裂期与中心体和微管存在共定位。
我们发现ITCH在结直肠肿瘤中显著的高表达,其泛素化活性受到MLN4924的抑制。为了探索靶向ITCH 的shRNA和 MLN4924对肿瘤细胞的发展有何作用,我们利用携带ITCH-sh-RNA的慢病毒体颗粒感染HCT116细胞后检测其增殖和迁移的能力,并检测在这些细胞系中MLN4924的作用。我们发现,靶向ITCH 的shRNA可以显著抑制结直肠细胞系的增殖和运动能力,且与MLN4924存在协同作用(Fig. AB)。
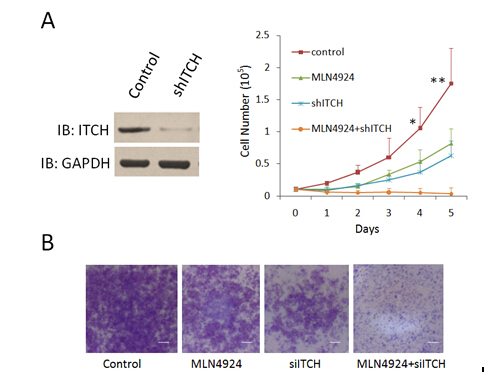
Fig.7 ShRNA targeting ITCH depressed the growth and metastasis of colorectal cancer cell line in vitro. (A)Lentivirus-coupled shRNA ITCH and control shRNA vectors were each stably transfected into HCT116 cells. The expression of ITCH was detected by western blot to show the depletion efficiency (lane 2). *p < 0.01, **p < 0.001 (unpaired two-tail t test). (B) The in vitro cell migration assay was performed in transwell plate. Representative results from three independent experiments are shown, bars, 100um.
3讨论
靶向泛素-蛋白酶体系统的抑制剂Velcade,目前已经用于治疗多发性骨髓瘤和淋巴瘤,效果良好。同一个公司开发的通过靶向Neddylation过程治疗结直肠癌的新药MLN4924,疗效更为显著,不良反应更小,已经进入II期临床实验,很可能成为新一代的重磅炸弹新药。前期的研究发现MLN4924能够抑制Culling类的泛素连接酶活性,上调周期调控因子,使肿瘤细胞周期阻滞,促进凋亡,从而达到治疗目的[12, 17, 21]。近期有报道Neddylation与Ubiquitination的交叉可能远比已知的要多,那么MLN4924在抑制Neddylation过程时,就会影响更广泛的泛素化过程。
我们发现ITCH具有Neddylation连接酶活性,并揭示了Nedd8对ITCH泛素化连接酶活性的调控作用。在结直肠肿瘤中ITCH高水平表达,在分裂期定位于中心体,提示ITCH很可能参与了肿瘤细胞的周期发展和调控。在结直肠肿瘤细胞系中敲低ITCH与MLN4924的作用一致,都能抑制肿瘤细胞的增殖和迁移,且ITCH的干扰RNA可起到MLN4924的协同作用,同时使用,大大增强了对肿瘤细胞的抑制作用。在泛素化反应的体系中,泛素连接酶具有底物识别的特异性,从而有可能成为肿瘤靶向性治疗的更好的靶标。因此,我们的研究不仅发现了MLN4924抑制肿瘤发展的一种新机制,而且为设计抗结直肠肿瘤药物提供了新的潜在药物靶标。
参考文献
1. Neefjes J, Groothuis TA, Dantuma NP: [The 2004 Nobel Prize in Chemistry for the discovery of ubiquitin-mediated protein degradation]. Ned Tijdschr Geneeskd 2004, 148(52):2579-2582.
2. Haas AL, Warms JV, Hershko A, Rose IA: Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem 1982, 257(5):2543-2548.
3. Thrower JS, Hoffman L, Rechsteiner M, Pickart CM: Recognition of the polyubiquitin proteolytic signal. EMBO J 2000, 19(1):94-102.
4. Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL: Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J 2003, 34(6):753-767.
5. Hershko A: The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ 2005, 12(9):1191-1197.
6. Chen C, Seth AK, Aplin AE: Genetic and expression aberrations of E3 ubiquitin ligases in human breast cancer. Mol Cancer Res 2006, 4(10):695-707.
7. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487(7407):330-337.
8. Oberst A, Rossi M, Salomoni P, Pandolfi PP, Oren M, Melino G, Bernassola F: Regulation of the p73 protein stability and degradation. Biochem Biophys Res Commun 2005, 331(3):707-712.
9. Salah Z, Melino G, Aqeilan RI: Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res 2011, 71(5):2010-2020.
10. Rossi M, De Simone M, Pollice A, Santoro R, La Mantia G, Guerrini L, Calabro V: Itch/AIP4 associates with and promotes p63 protein degradation. Cell Cycle 2006, 5(16):1816-1822.
11. Suryaraja R, Anitha M, Anbarasu K, Kumari G, Mahalingam S: The E3 ubiquitin ligase Itch regulates tumor suppressor protein RASSF5/NORE1 stability in an acetylation-dependent manner. Cell Death Dis 2013, 4:e565.
12. Schulman BA, Harper JW: Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol 2009, 10(5):319-331.
13. Whitby FG, Xia G, Pickart CM, Hill CP: Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J Biol Chem 1998, 273(52):34983-34991.
14. Gong L, Yeh ET: Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem 1999, 274(17):12036-12042.
15. Liu J, Furukawa M, Matsumoto T, Xiong Y: NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases. Mol Cell 2002, 10(6):1511-1518.
16. Zheng J, Yang X, Harrell JM, Ryzhikov S, Shim EH, Lykke-Andersen K, Wei N, Sun H, Kobayashi R, Zhang H: CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Mol Cell 2002, 10(6):1519-1526.
17. Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO: Targeting the ubiquitin-proteasome system for cancer therapy. Cancer Sci 2009, 100(1):24-28.
18. Eldridge AG, O'Brien T: Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ 2010, 17(1):4-13.
19. Berkers CR, Ovaa H: Drug discovery and assay development in the ubiquitin-proteasome system. Biochem Soc Trans 2010, 38(Pt 1):14-20.
20. Nalepa G, Rolfe M, Harper JW: Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 2006, 5(7):596-613.
21. Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S et al: An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 2009, 458(7239):732-736.
22. Leidecker O, Matic I, Mahata B, Pion E, Xirodimas DP: The ubiquitin E1 enzyme Ube1 mediates NEDD8 activation under diverse stress conditions. Cell Cycle 2012, 11(6):1142-1150.
23. Hjerpe R, Thomas Y, Kurz T: NEDD8 overexpression results in neddylation of ubiquitin substrates by the ubiquitin pathway. J Mol Biol 2012, 421(1):27-29.
24. Hjerpe R, Thomas Y, Chen J, Zemla A, Curran S, Shpiro N, Dick LR, Kurz T: Changes in the ratio of free NEDD8 to ubiquitin triggers NEDDylation by ubiquitin enzymes. Biochem J 2012, 441(3):927-936.
25. Ishihara T, Inoue J, Kozaki K, Imoto I, Inazawa J: HECT-type ubiquitin ligase ITCH targets lysosomal-associated protein multispanning transmembrane 5 (LAPTM5) and prevents LAPTM5-mediated cell death. J Biol Chem 2011, 286(51):44086-44094.
北京药学会 地址:北京市朝阳区北三环中路2号小二楼2层
本网站浏览52127919次
Copyright 2012 北京药学会( 本网站所有内容未经许可,不得以任何形式进行转载 ) All Rights Reservered